Molecule Systematic Names and Common Names
What are Molecule Systematic Names and Common Names?
✍: FYIcenter.com
![]() Molecule Systematic Names are derived with a nomenclature system
for simple organic molecules.
Molecule Systematic Names are derived with a nomenclature system
for simple organic molecules.
Some rules used by the nomenclature system for binary (two-element) molecules are:
- The more electropositive atom is written first and the more electronegative element is written last with an -ide suffix.
- The Greek prefixes are used to dictate the number of a given element present in a molecular compound.
- Prefixes can be shortened when the ending vowel of the prefix “conflicts” with a starting vowel in the compound.
Here is the list of Greek prefixes used in the nomenclature system:
Number of Greek
Elements Prefix
--------- ------
1 mono- (not used on the first element)
2 di-
3 tri-
4 tetra-
5 penta-
6 hexa-
7 hepta-
8 octa-
9 nona-
10 deca-
Examples of systematic names for binary molecules are:
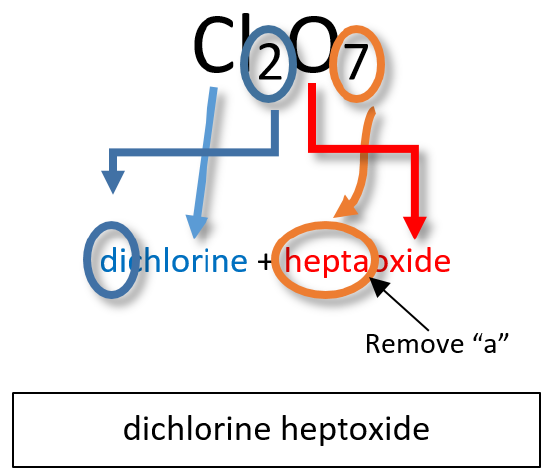
Systematic Names Formula -------------------- ------- Dihydrogen Monoxide H₂O Carbon Dioxide CO₂ Nitrogen Monoxide NO Dinitrogen Monoxide N₂O Disulfur Dichloride S₂Cl₂ Dichlorine Heptoxide Cl₂O₇
The diagram below shows you how the systematic name Dichlorine Heptoxide is derived for Cl₂O₇:
Molecule Common Names are commonly used names for some popular molecules.
Examples of common names are:
Common Names Formula --------------- ------- Water H₂O Ethanol C₂H₆O
⇒ Brand Names vs. Generic Names for Drugs
⇐ Molecule Presentation and Identification
⇑ Molecule Presentation and Identification
⇑⇑ Molecule FAQ
2023-01-24, 1094🔥, 0💬