What Is Radical Molecule
What is Radical Molecule?
✍: FYIcenter.com
![]() A Radical molecule, also called free radical, is molecule that has one or more radical centers.
A Radical molecule, also called free radical, is molecule that has one or more radical centers.
A Radical center is an atom in a molecule that has an unpaired valence electron, or odd number of valence electrons (including both bounded and unbound electrons).
When writing the chemical formula for a radical molecule, its radical atoms are identified with superscript dots "·".
Example 1: HO· - Hydroxyl radical has one radical center at the O atom, which has 7 valence electrons, with 4 paired and unbounded electrons, 2 paired and bounded electrons, and unpaired electron. If a hydroxyl radical loses the unpaired eletron, it becomes an positive ion, called hydroxyl cation. If a hydroxyl radical gains an extra eletron, it also becomes an negative ion, called hydroxyl anion.
Example 2: H3C· - Methyl radical has one radical center at the C atom, which has 7 valence electrons, with 6 paired and bounded electrons and 1 unpaired electron. If a methyl radical loses the unpaired eletron, it becomes an positive ion, called methyl cation. If a hydroxyl radical gains an extra eletron, it also becomes an negative ion, called methyl anion.
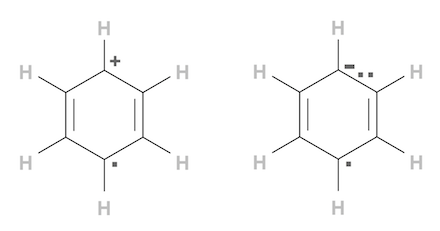
Example 3: H6C6·- - Benzene radical anion has one radical center at a C atom, which has 7 valence electrons, with 6 paired and bounded electrons and 1 unpaired electron. The extra negative charge is coming from another C atom which gained an extra election.
Example 4: H6C6·+ - Benzene radical cation has one radical center at a C atom, which has 7 valence electrons, with 6 paired and bounded electrons and 1 unpaired electron. The extra positive charge is caused by another C atom which lost an election.
Â
⇒ What Is Isomer
⇑⇑ Molecule FAQ
2020-08-03, 3329🔥, 1💬