What Are Valence Electrons
What Are Valence Electrons of an Atom?
✍: FYIcenter.com
![]() Valence Electrons of an Atom are electrons located on the most outer shell
of the atom.
Valence Electrons of an Atom are electrons located on the most outer shell
of the atom.
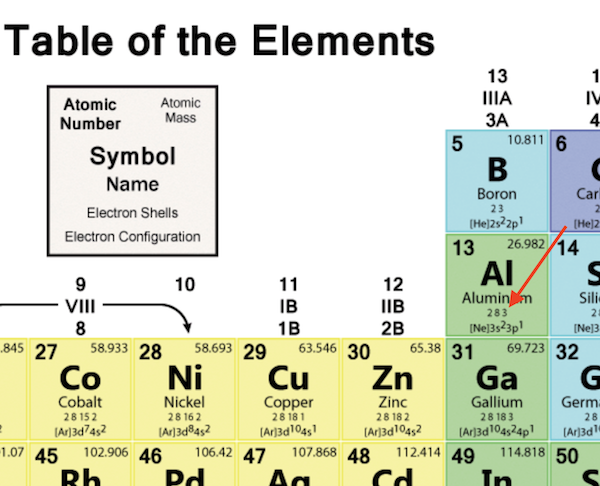
The number of Valence Electrons of an atom is very important chemical property of the atom. There is no easy way to calculate the number of Valence Electrons of an atom. But you can can easily find the number of Valence Electrons of an atom from the periodic table.
To simply the task, you should be a periodic table that has electron shells and electron configuration.
For example, the periodic table below shows you that the number of valence electrons of the alumium atom is 3.
Â
⇠What Is Ion
⇑⇑ Molecule FAQ
2020-05-29, 4645🔥, 0💬