What Is Formal Charge
What is Formal Charge of an atom in a molecule? And what is the total formal charge of a molecule?
✍: FYIcenter.com
![]() The formal charge of an atom in a molecule is defined by the formula below:
The formal charge of an atom in a molecule is defined by the formula below:
Fc (Formal charge) of atom = Ve - Ue - Be/2 where: Ve = number of valence electrons Ue = number of unbonded electrons Be = number of bonded electrons
The formal charge of a molecule is defined by the formula below:
Formal charge of molecule = Sum of formal charges of all atom
The best way to calculate the formal charge of an atom in a molecule is to present the molecule in the Lewis structure format. The calculation can be divided into 4 steps:
Step 1: Look up "Ve (valence electrons)" from the periodic table for atom. Step 2: Count dots around the atom as "Ue (unbonded electrons)". Step 3: count bound lines connected to the atom as "Be (bonded electrons) / 2". step 4: Calculate "Fc (Formal charge)" as Ve - Ue - Be/2.
Example 1 - Hydrogen Isocyanide: Both Lewis Structure and Skeletal Formula of Hydrogen Isocyanide molecule are shown below:
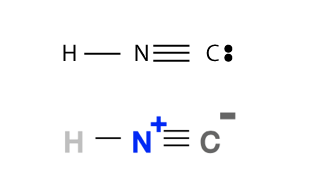
From the Lewis Structure in the above picture, you can easily calculate the formal charge of each atom in the molecule and the formal charge of the molecule itself:
Fc of H = 1 - 0 - 1 = 0 Fc of N = 5 - 0 - 4 = 1 Fc of C = 4 - 2 - 3 = -1 Fc of molecule = 0 + 1 + -1 = 0
So for Hydrogen Isocyanide, the entire molecule is ectrically neutral with 0 formal charge. But the N atom has a formal charge of 1, losing 1 electron to the neighbor atom C; which has a formal charge of -1 because of this extra electron.
Atom's formal charge is usually included in the Skeletal Formula as shown in the above picture.
Example 2 - Carbonate Anion: Both Lewis Structure and Skeletal Formula of Carbonate Anion are shown below:
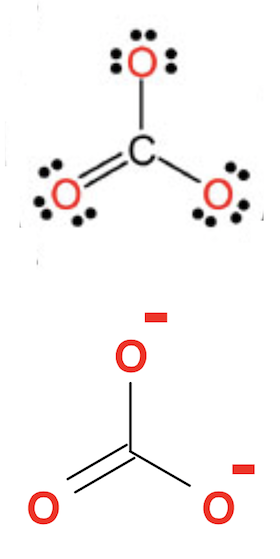
From the Lewis Structure in the above picture, you can easily calculate the formal charge of each atom in the molecule and the formal charge of the molecule itself:
Fc of C = 4 - 0 - 4 = 0 Fc of O = 6 - 4 - 2 = 0 Fc of O = 6 - 6 - 1 = -1 Fc of O = 6 - 6 - 1 = -1 Fc of molecule = 0 + 0 + -1 + -1 = -2
So for Carbonate Anion, the entire molecule has a formal charge of -2, coming from two carbon atoms, each of them has a formal charge of -1. Those 2 extra electrons comes from outside of the anion.
Â
⇠What Are Valence Electrons
⇑⇑ Molecule FAQ
2020-05-29, 2630🔥, 0💬